News
Press releases, workshop summaries, and editorials
NINDS Parkinson's Disease Biomarkers Program Consortium Meeting, Virtual (October 16, 2024)
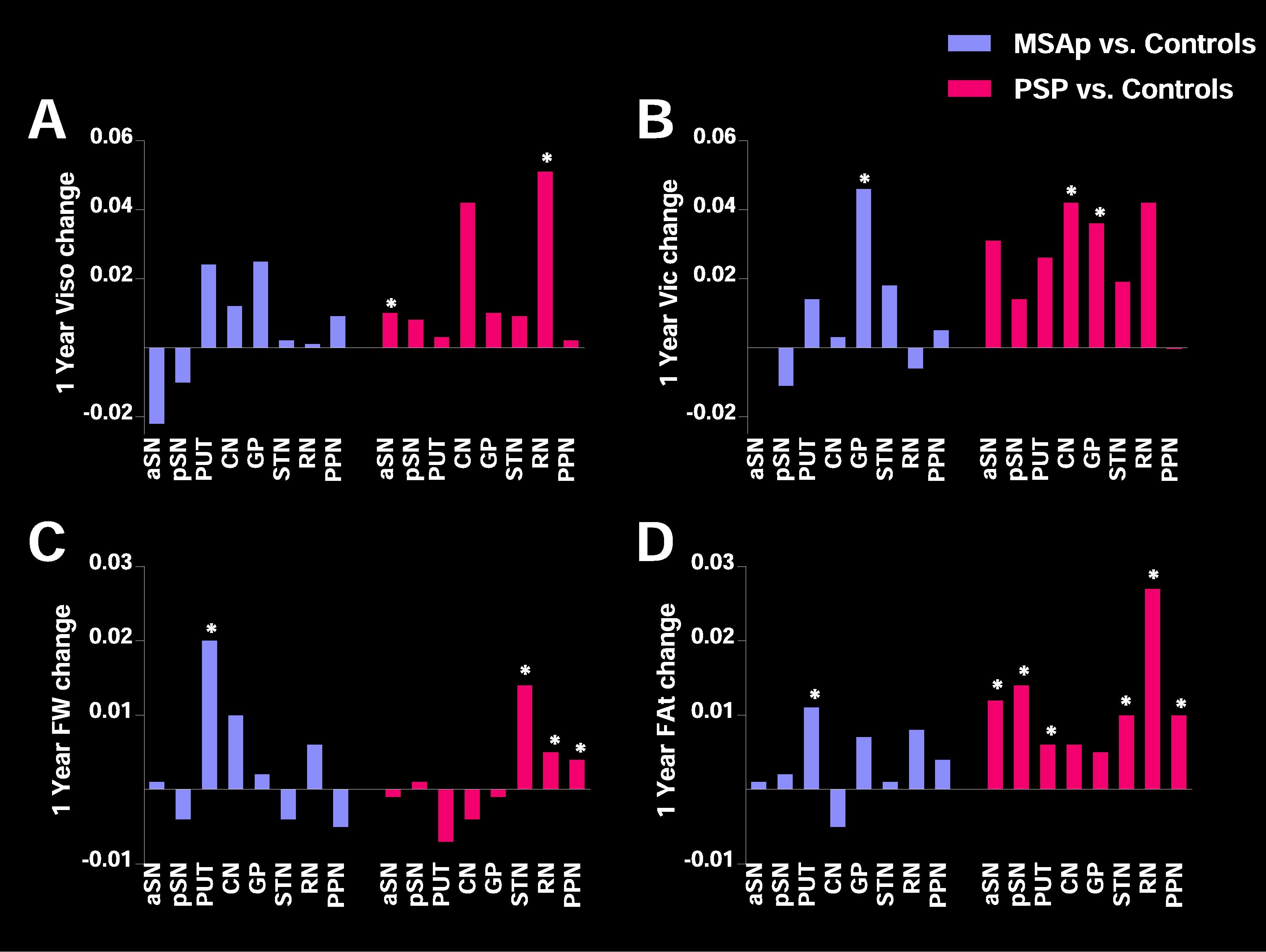
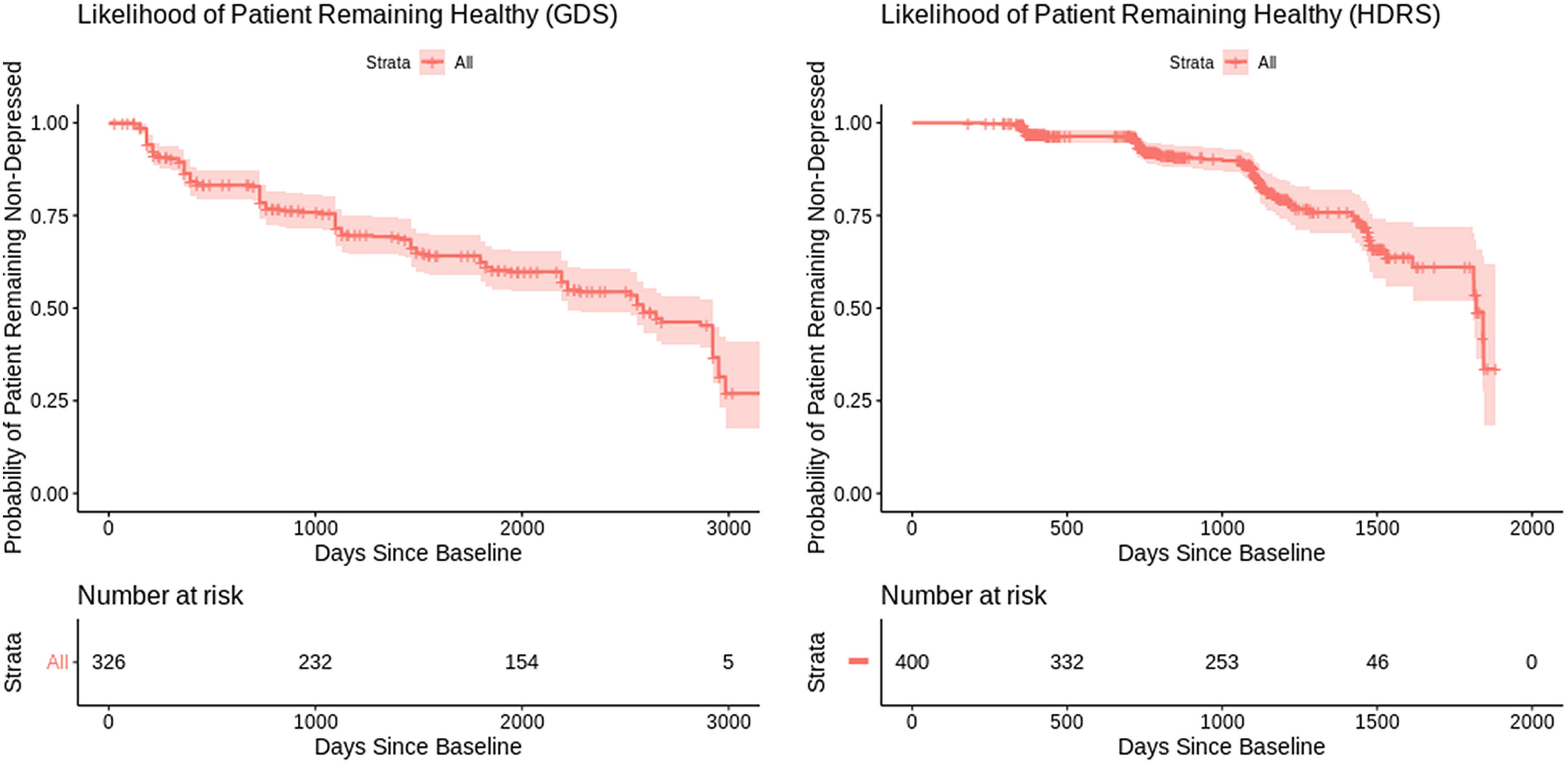
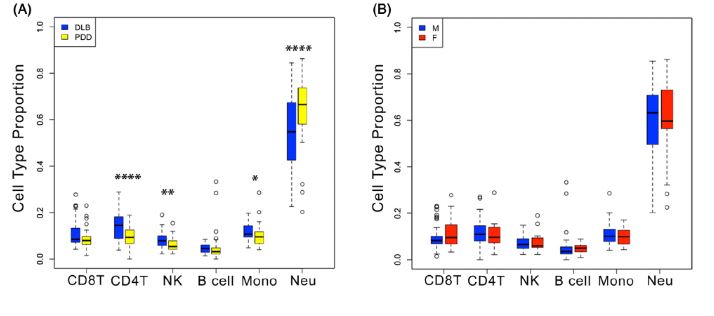
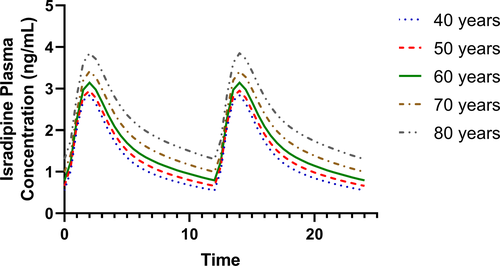
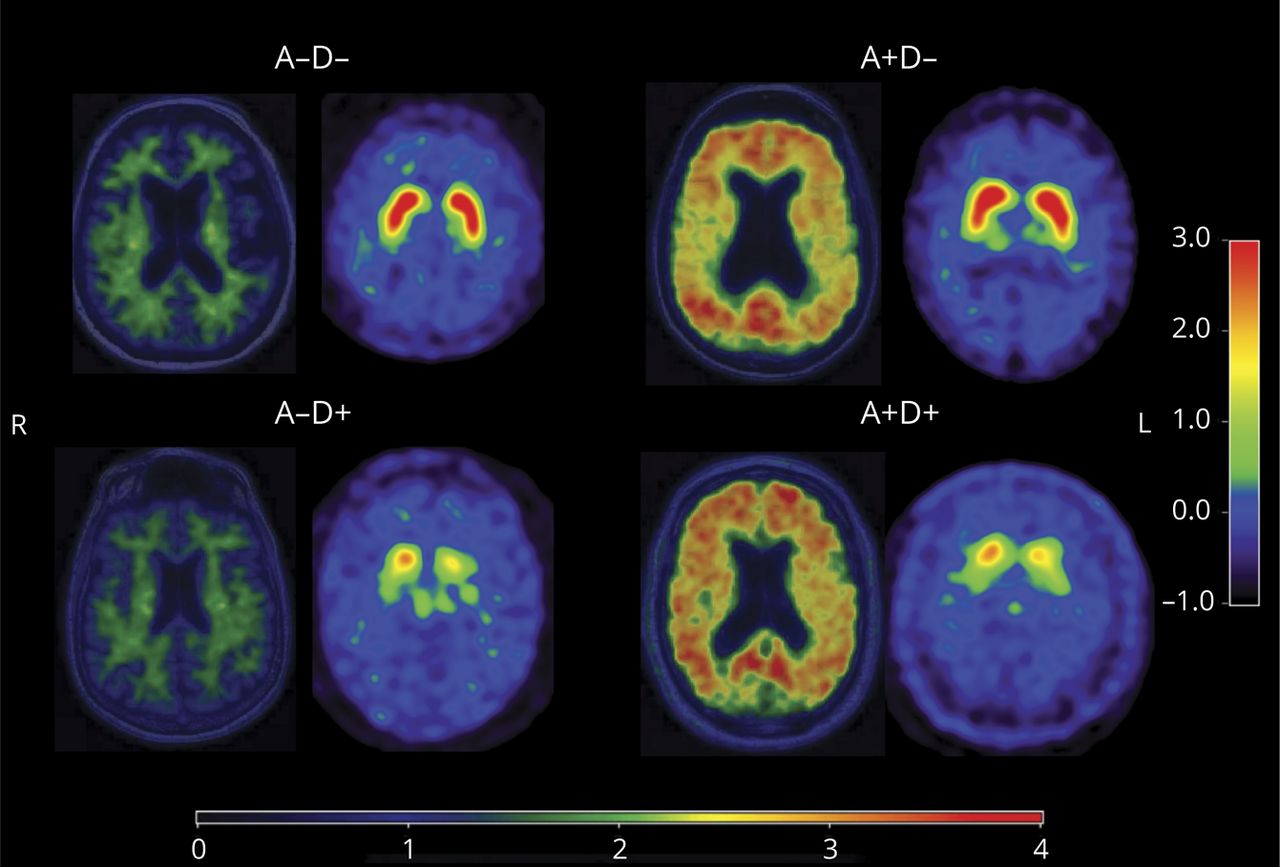
The 2024 NINDS Parkinson’s Disease Biomarkers Program (PDBP) Annual Meeting provided a forum for participating scientists and stakeholders to discuss recent progress and emergent opportunities in Parkinson’s disease (PD), Lewy body dementia (LBD), and related parkinsonism biomarkers research.
NINDS Parkinson's Disease Biomarkers Program Consortium Meeting, Bethesda MD (September 21, 2023)
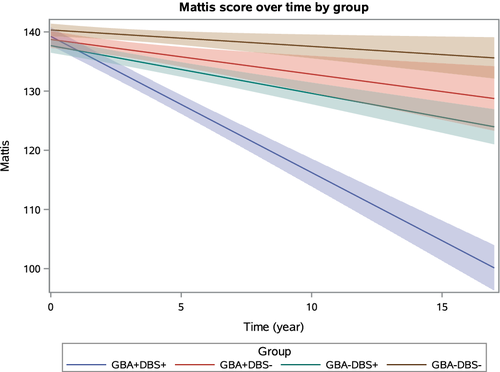
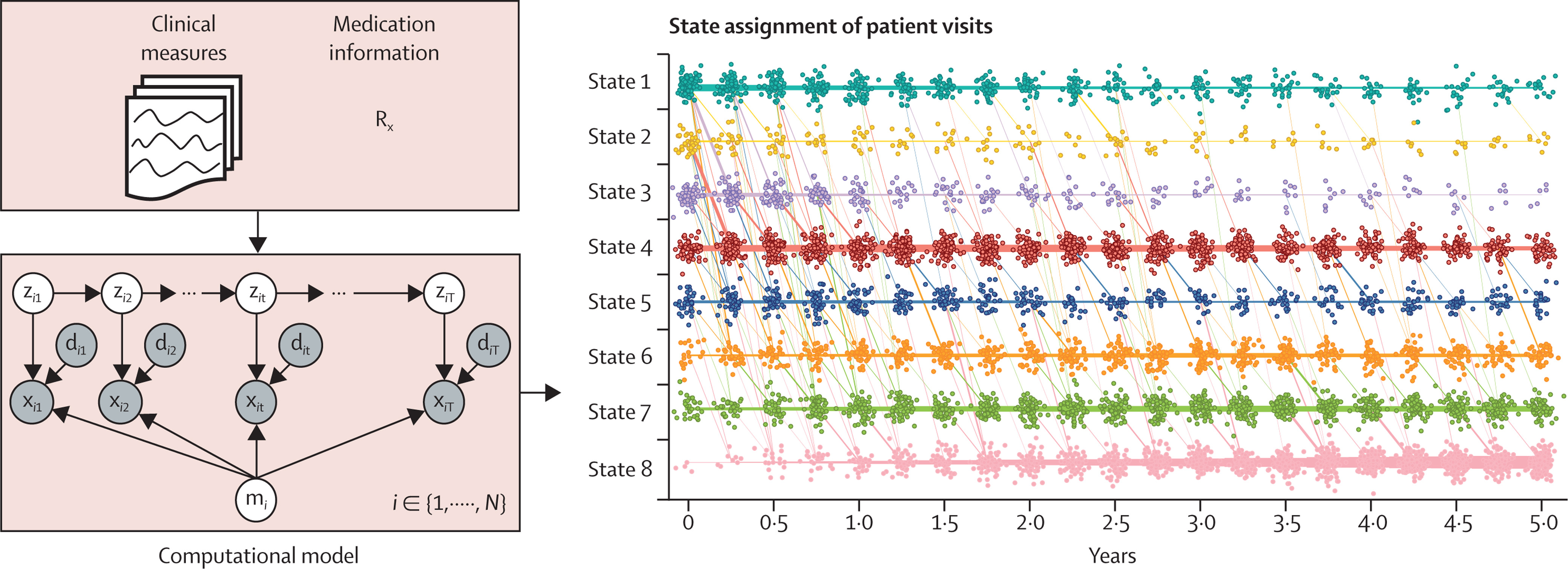
The 2023 NINDS PDBP Annual Meeting (hybrid) was held on September 21, 2023. Principal investigators, clinical coordinators and data management experts from each PDBP project, persons with lived experience, as well as representatives from the National Institutes of Health (NIH) and PD nonprofit organizations met to discuss biomarkers research and progress made over the last twelve months.