Objectives
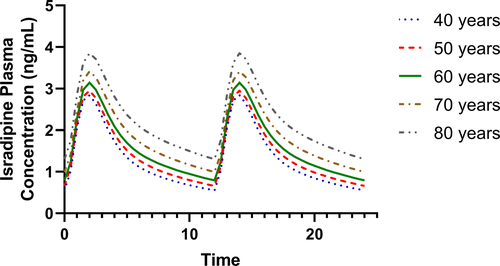
Isradipine is a dihydropyridine calcium channel inhibitor that has demonstrated concentration‐dependent neuroprotective effects in animal models of Parkinson’s disease (PD) but failed to show efficacy in a phase 3 clinical trial. The objectives of this study were to model the plasma pharmacokinetics of isradipine in study participants from the phase 3 trial; and, to investigate associations between drug exposure and longitudinal clinical outcome measures of PD progression.
Methods
Plasma samples from nearly all study participants randomized to immediate‐release isradipine 5‐mg twice daily (166 of 170) were collected for population pharmacokinetic modeling. Estimates of isradipine exposure included apparent oral clearance and area under the concentration‐time curve. Isradipine exposure parameters were tested for correlations with 36‐month changes in disease severity clinical assessment scores, and time‐to‐event analyses for initiation of antiparkinson therapy.
Results
Isradipine exposures did not correlate with the primary clinical outcome, changes in the antiparkinson therapy‐adjusted Unified Parkinson’s Disease Rating Scale parts I–III score over 36 months (Spearman rank correlation coefficient, rs: 0.09, P = 0.23). Cumulative levodopa equivalent dose at month 36 was weakly correlated with isradipine plasma clearance (rs: 0.18, P = 0.035). This correlation was sex dependent and significant in males, but not females. Those with higher isradipine exposure had decreased risk of needing antiparkinson treatment over 36 months compared with placebo (hazard ratio: 0.87, 95% CI: 0.78–0.98, P = 0.02).
Interpretation
In this clinical trial, higher isradipine plasma exposure did not affect clinical assessment measures of PD severity but modestly decreased cumulative levodopa equivalent dose and the time needed for antiparkinson treatment initiation.
Source: https://onlinelibrary.wiley.com/doi/10.1002/acn3.51300
DOI: https://doi.org/10.1002/acn3.51300