NINDS Centralized GUID Server
The National Institute of Neurological Disorders & Stroke (NINDS), in conjunction with the National Institute of 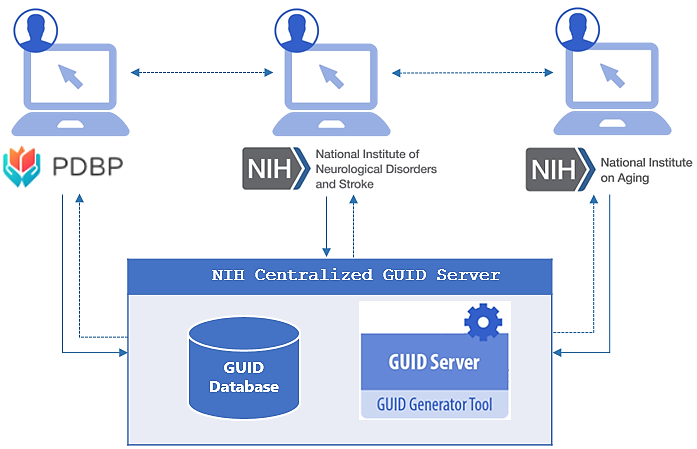 Aging (NIA), and the Parkinson’s Disease Biomarkers Program (PDBP), have successfully collaborated to develop a Multi-Tenant ‘Global Unique Identifier’ (GUID) solution for participant sharing across institutes and studies, known as the NINDS Centralized GUID solution.
Aging (NIA), and the Parkinson’s Disease Biomarkers Program (PDBP), have successfully collaborated to develop a Multi-Tenant ‘Global Unique Identifier’ (GUID) solution for participant sharing across institutes and studies, known as the NINDS Centralized GUID solution.
The GUID, as part of the Biomedical Research Informatics Computing System (BRICS) platform, allows data to be associated with a research participant without exposing or transferring personally identifiable information (PII) and/or protected health information (PHI). Expanding on this concept, the NINDS Centralized GUID solution offers authorized researchers from PDBP, NINDS, and NIA new capabilities, including:
1. Management of study participants through the generation of a secure, random alphanumeric identifier (GUID)
2. Mapping of study participants to study data for more efficient/comprehensive analysis
3. Visibility to matching study participants/GUIDs across institutes and studies
4. Collaboration amongst researchers to share study data (offline) about participants
Overview of the GUID General Process:
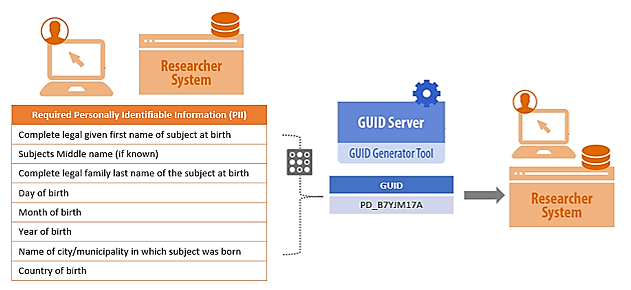
BRICS GUIDs are generated using specific participant PII/PHI and converted into a series of hash-codes using a specialized algorithm. The software generates hash-codes (one-way encryption) on the researcher’s machine, the hash-codes are then sent to the GUID server; at no point does any PII/PHI leave the researcher’s machine. This one-way encryption ensures that the GUID cannot be used to recreate any portion of the original PII/PHI. A video tutorial about the GUID generation process can be found here: GUID Generation Process.
Once the NINDS Centralized GUID server receives the hash-codes, a check is performed for the following conditions:
1. Does the GUID already exist for a PDBP, NINDS, or NIA, study participant?
2. If the GUID already exists, can it be accessed by the user?
Depending on the initial condition check, an existing GUID will be returned to the registered user, along with meta-data including: 1) who originally registered the GUID (last name & first name), 2) what institute registered the GUID (NINDS, NIA, or PDBP), and 3) the date that the GUID was registered. No study data or information pertaining to a study is ever accessible through the NINDS Centralized GUID solution. Registered users who want additional information about a GUID(s) must formally request access with the appropriate institute/study that stores the data associated with the GUID(s), and apply for specific data access approval processes. The intent is to facilitate communication between NINDS, NIA, and PDBP researchers, while ensuring that regulation and/or policy is not circumvented and study data is not inadvertently shared without proper approval.
If the hash-codes do not result in a match, a new GUID (a random 10-digit alpha-numeric format: NIH012ABC34DE) is generated which is then linked to the study participant and universally shared across all of NINDS, NIA, and PDBP. Moving forward, any researcher that inputs the same participant PII/PHI will receive the universal GUID.
Questions & Resources:
NINDS program staff will identify NINDS clinical studies where access to the Centralized GUID Server will facilitate data and biospecimen sharing. In exceptional circumstances, access to the GUID server will be provided to non-NINDS funded projects; please contact NINDS staff for access.
For additional information about getting access to either the NINDS Centralized GUID Solution, please contact the PDBP Operations email: PDBP-OPS@mail.nih.gov. Visit the following sites to request a user account and begin generating GUIDs: